Pretty Solar Spectra
Tell the story of why we care about ozone ...
Following on from my last post …. There are about 1000 words in this one. It’s a good example of a picture being worth a thousand words.
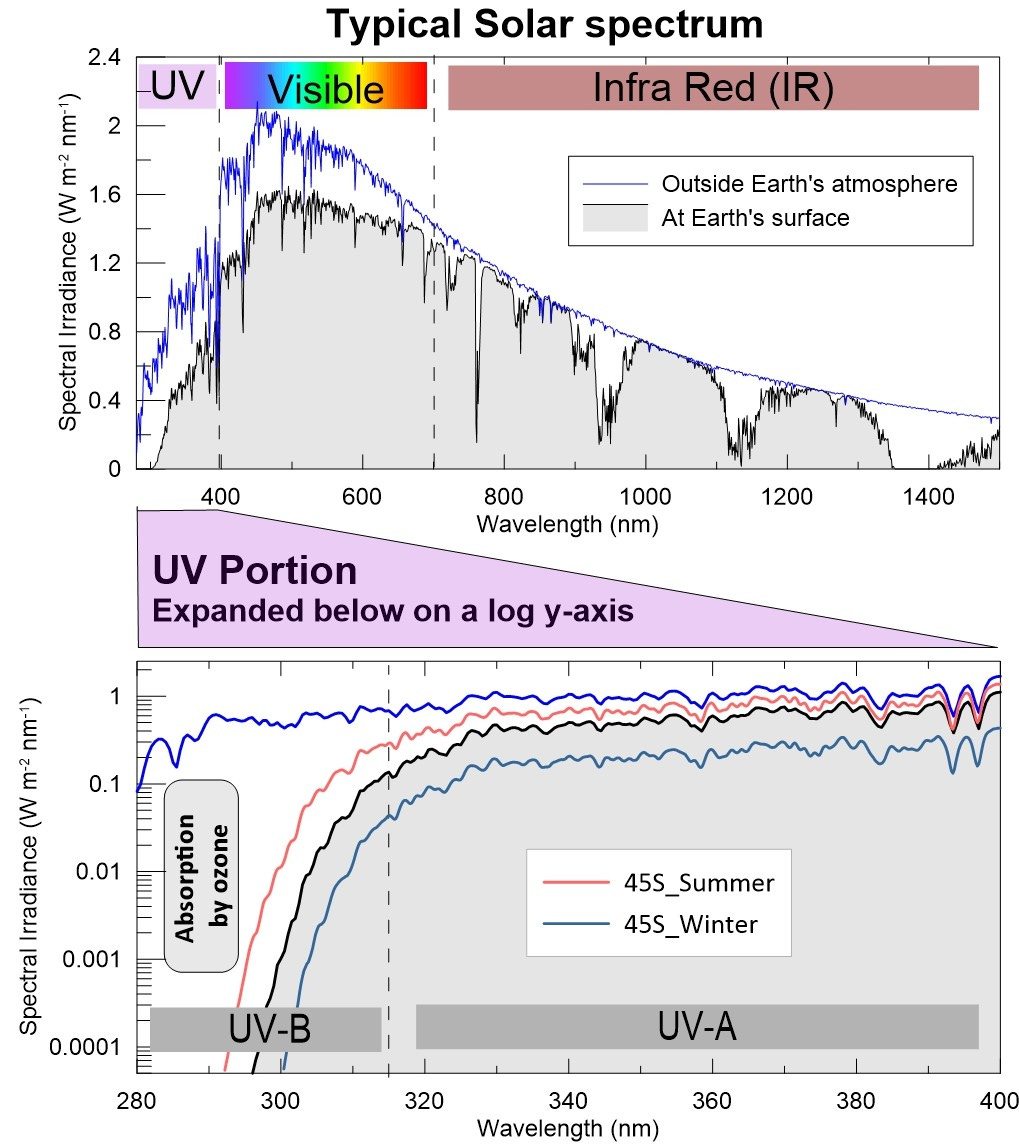
I made a pretty plot the other day that I thought I’d share. It’s really two plots, as shown below. Take a closer look before I go into the details. Together they tell us a lot about why we care about ozone.
The upper panel compares the extra-terrestrial (ET) spectrum of sunlight outside Earth’s atmosphere (blue line) with a reference spectrum at Earth’s surface (black line) that’s used by the solar energy community in the USA. It’s called the “ASTM G173-03 energy reference spectrum”, which represents the annual mean solar energy available at noon in the contiguous USA and is calculated for airmass 1.5 (i.e, for a sun elevation about 48 degrees above the horizon), with the incident surface tilted 37 degrees towards the sun, as would be typical for a solar panel. Coincidentally (or perhaps not?), the total electrical energy generation capability from this spectrum - the power available - is very close to 1 kW per square metre (compared with about 1.4 kW per square meter in the ET spectrum). That 1 kW number is a nice benchmark. It’s the same as the energy consumption from an old 1-bar electric heater, though with current technology only around 20 percent of that can be realised in practice.
The spectra extend much further out into the infra-red (IR) region, but I’ve included wavelengths only up to 1500 nm (1.5 microns) in the near infra-red. More than half the total solar total energy is at infra-red wavelengths. The visible portion of the spectrum which extends from violet (400 nm) to red (700 nm) is highlighted with the colours of the rainbow. The energy falling within this most-familiar component is only about 40 percent of the total. The UV region - at wavelengths less than 400 nm - contributes around 7 percent of the total.
The sharp features in the ET spectrum (blue line) are absorptions from gases in the Sun’s atmosphere - called ‘Fraunhofer’ absorption lines, named after their discoverer. The same features of course also occur in the spectrum received at Earth’s surface (black line), which falls progressively below the ET spectrum at shorter wavelengths because of the scattering losses discussed previously. Effets of absorption by gases in the Earth’s atmosphere can also be seen in the UV and IR regions. The large absorption features at longer wavelengths are mainly due to water vapour in the Earth’s lower atmosphere, while absorption by ozone higher in the atmosphere become progressively more important at shorter UV wavelengths.
With this linear scaling of the y-axis, it’s hard to see the small UV-B contribution (wavelengths from 280 to 315 nm) that reaches the surface. In fact, it looks to be essentially zero at all wavelengths less than around 300 nm. But these wavelengths are damaging to life because their photon energies are sufficient to break molecular bonds, including those in molecules of DNA. Because of the importance of radiation from those short wavelengths, UV spectra are usually plotted instead on the logarithmic y-axis (explained below).
The lower panel above is an expansion of just the UV component in the upper panel, this time plotted on a logarithmic y-axis to highlight differences at the shortest wavelengths. Each major division on the y-axis now represents a factor of ten difference, and the smallest number shown is just one ten-thousandth of the largest. The non-linearity of the scale can also be seen in the minor tick marks. Although the divisions are equal, with the log y-axis they become more and more crowed at larger values within each major interval.
While the ET spectrum remains fairly constant over the UV-B range, decreasing by about a factor of 2 from 315 to 290 nm; that at Earth’s surface drops like a stone towards shorter wavelengths, and is already thousands of times smaller at 295 nm than at 315 nm. That huge reduction in the UV-B region is due to absorption by ozone - mainly in the stratosphere at altitudes from 15 to 30 km. It does a great job of protecting us!
Also shown in the lower plot are two spectra measured near my home in New Zealand, at NIWA’s Lauder atmospheric research laboratory (45 S, 170 E, alt. 370 m). They are typical for noon on clear days close to the summer and winter solstices. The detailed structure that’s apparent - especially in the UV-A region of the measured spectra - is exactly as expected. But, unlike the ET spectrum, all three spectra at Earth’s surface plummet at shorter UV-B wavelengths. You can see that at 300 nm, the contribution in summer is about 200 times larger than in winter, and that ratio becomes larger at shorter wavelengths and smaller at longer wavelengths.
The damaging effect on human skin extends over a range of UV wavelengths, and in sunlight the most important are in the UV-B region peaking between 305 and 310 nm. It turns out that sunburning UV is about ten times greater for the summer spectrum shown (UVI = 11.5) than for the winter spectrum (UVI = 1). The maximum UVI anywhere on Earth’s surface is ~ 25 (at high altitudes in the tropics), but outside Earth’s atmosphere, it would be more than an order of magnitude higher than that (i.e., UVI = 300!!). Instead of skin damage occurring in a few minutes, it would take seconds.
We have ozone to thank for enabling life as we know it to thrive on Planet Earth.
Incidentally, I was curious to see if the calculated spectrum (black line) actually included diffuse skylight, or if it was only for the direct beam, which dominates at the visible and near-infrared wavelengths relevant to solar panels. I checked the calculation parameters and convinced myself that they had indeed included both scattered and direct components. But, if you compare the slopes of that calculated spectrum with the two measured spectra, you can see that throughout the UV-A region, the calculated spectrum drops off towards shorter wavelengths more quickly than the measured spectra. That’s because, with the surface being tilted towards the sun it doesn’t see the entire sky so there’s a smaller contribution from its bluer skylight.
Thanks for reading this. Previous posts on the intersection between Ozone, UV, Climate, and Health can be found at my UV & You area at Substack.