We keep hearing about the dangers of UV-B radiation, but never much about UV-C. Why?
The spectrum of UV radiation is usually split arbitrarily into three sub-bands:
UV-A: the longest wavelength range (315 to 400 nm), which is the most prevalent and least damaging,
UV-B: an intermediate wavelength range (280 to 315 nm), which is more damaging but less plentiful, mainly because it’s so efficiently absorbed by ozone (and glass), and
UV-C: the shortest wavelength range (100 to 280 nm), which would be the most damaging, but none is transmitted to Earth’s surface.
For that reason, human health advisors rarely mention UV-C, though it is an important driver of atmospheric chemistry higher in the atmosphere.
The shorter the wavelength, the more energy each photon of radiation carries. With its higher photon energies, each photon of UV-C is undoubtedly more dangerous than UV-B. Interestingly though, the action spectra for damage (e.g., causing skin reddening, or ‘erythema’) often show no increase at UV-C wavelengths - possibly because since no UV-C from sunlight ever reaches Earth’s surface nobody has bothered to assess its impact at those wavelengths.
But there are a few UV-C sources that we do need to be concerned about. The main ones are arc welders commonly used by engineers; and a few specialist discharge lamps that are used mainly in controlled laboratory conditions (e.g., in optical experiments, or as a curing agent). It’s also present in old mercury vapour street lamps that are rapidly being superseded by more modern LED lamps. By far the strongest emission from those old lamps is from the UV-C region, at a wavelength near 254 nm. But we don’t see any of that because those wavelengths are not transmitted by their glass envelopes. It does - however - contribute to the fluorescence from the coatings deposited on the inner walls of those glass envelopes. If the lamp doesn’t have a fluorescent coating, it will be much less efficient, and the main light we see is from an atomic emission line near 436 nm which gives its bluish colour. Because of its damaging effect on DNA (and RNA), UV-C is also an antiseptic agent, such as when it’s used irradiate household water supplies to kill bacteria.
So, why don’t we have to worry about UV-C from sunlight?
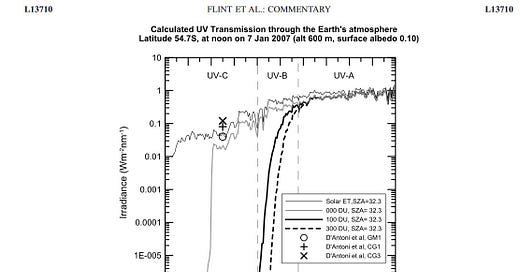
Firstly, the amount emitted by the Sun is very small, much lower than at visible wavelengths. The graph below shows that outside Earth’s atmosphere, the solar radiation at 200 nm is an order of magnitude less than that at 280 nm (which, as I showed a couple of weeks back, is already only as small fraction of the maximum). Secondly, we know from laboratory studies that ozone and other gases present in the atmosphere absorb most of that. Finally, our own measurements using the best UV spectrometers of their type in the world show that there’s no detectable signal at wavelengths less than about 290 nm, even at the high altitude Mauna Loa Observatory in Hawaii (latitude 19.5N, altitude 3.4 km), where the maximum UV levels reached (UVI > 21) are close to the world’s highest.
It’s true that investigators occasionally claim to have measured high amounts of UV-C at the surface, but it always turns out that they’ve misinterpreted their results because their equipment was ill-suited to the demanding task. It’s demanding because there so little of it that you need to take careful precautions to avoid contamination caused by stray light from longer wavelengths.
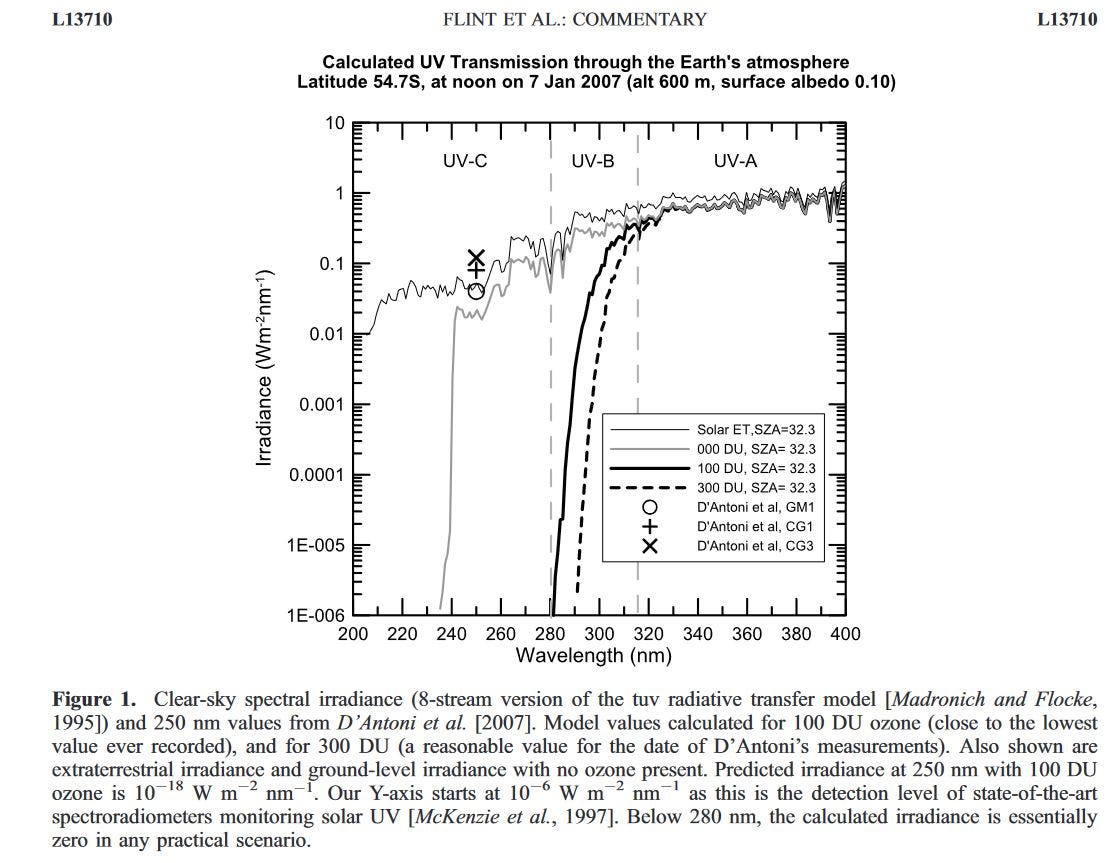
In one such case, back in 2008, we could easily disprove their results because the amounts of UV-C they reported exceeded the output from the Sun arriving outside Earth’s atmosphere at those wavelengths. Here’s the main figure from our paper debunking the work. It shows that one of their measurements near 250 nm was about 16 orders of magnitude higher that it should have been for those conditions, and an order of magnitude higher than it would have been if there were no ozone at all!
The plot doesn’t include wavelengths from 100 to 200 nm, but we know that don’t have to worry about that range. It’s called the ‘vacuum’ ultraviolet range because air absorbs so strongly there - even for short light paths - that you need to carry out optical studies under vacuum.
The steep cut-off below 250 nm - even when there is no ozone present (the grey curve) - is caused by absorption by molecular oxygen.
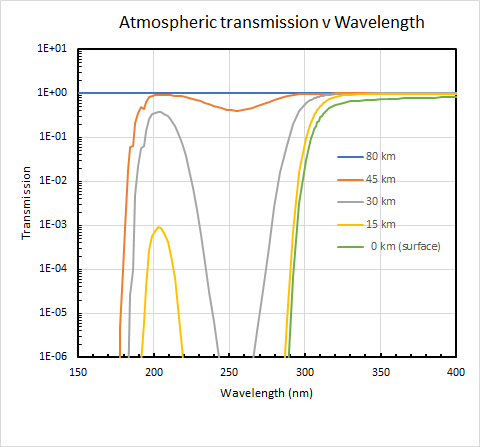
As shown by the figure below, some solar UV-C radiation can penetrate some distance into the atmosphere, but none gets as far as the Earth’s surface. At wavelengths less than 250 nm, absorption by atmospheric ozone start diminishing, but over that same wavelength range absorption by oxygen starts to increase. Because the peak absorption by oxygen is smaller than that of ozone, this leads to an atmospheric ‘window’ of sorts near 220 nm where solar UV-C radiation can penetrate deepest into the atmosphere. For example, the figure shows that for overhead sun about 40 percent at that wavelength penetrates to 30 km (grey curve - note the log y-axis), but only 0.1 percent penetrates down through the ozone layer to 15 km (yellow curve), and none of it reaches the surface (green curve).
That substantial amount of solar radiation reaching as far down as 30 km is an important contributor to photo-chemical reactions that help maintain the balance of ozone in the stratosphere. But down where we live, our skin is saved from its ravages - as long as we stay firmly anchored to Earth’s surface.
Thanks for reading this. Previous posts on the intersection between Ozone, UV, Climate, and Health can be found at my UV & You area at Substack.