Curly Questions about Carbon Dioxide
A for-once interesting question posed by a climate skeptic
I get a lot of questions from Ozone Skeptics and Climate Change Deniers. Usually it’s the same recurring “misunderstandings” from grumpy old white men with vested interests. Only very occasionally do they lead to anything interesting. But an article that crossed my desk this week did.
It was forwarded to me for clarification by a friend, and questioned (among the other usual things) one of the pillars of the standard climate change dogma. The article had been written by one of his clients, and claimed that the atmospheric lifetime of carbon dioxide (CO2) couldn’t really be as long as the hundreds of years we think it is. It argued that its lifetime must be much shorter, and therefore much less important.
As evidence, the authors pointed to the graph below, which shows the rise and decay of the “modern” isotope of carbon dioxide - that was produced in the 1950s and 1960s from nuclear testing in the atmosphere. That radioactive isotope of modern carbon (called radiocarbon, or Carbon 14) has the usual 6 protons, but two extra neutrons.
At first, the percentage of modern carbon relative to its 1955 value (labelled pMC in the graph) increased as a result of that nuclear testing. But, following the Partial Test Ban Treaty (PTBT), signed on 10 October 1963, it decayed back to its 1955 value with a half life of only a few decades; much shorter than the 300 to 1000 years usually quoted by climate scientists. They also argued that the modern carbon in this form of CO2 is the same in all respects as ordinary carbon (Carbon 12) which form the vast bulk of the gas in the atmosphere.
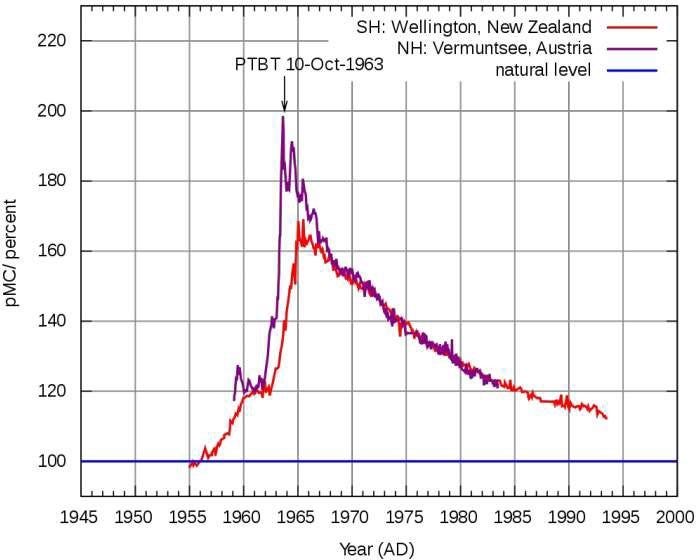
(Those Wellington measurements were continued by my NIWA colleagues for many years).
The plot does indeed raise some interesting questions, but their conclusion is incorrect. When climate scientists talk about the atmospheric lifetime, they mean something a bit more complicated. As the bomb-test data shows, an individual molecule of CO2 lasts only a few decades. But that’s not the end of its contribution in the atmosphere. Molecules of CO2 are constantly being taken up by plants during the day (photosynthesis) and re-emitted by plants and animals (respiration); with much larger fluxes in summer than winter. There are also large fluxes between the atmosphere and ocean.
These interactions are summarized in the figure below from an excellent paper in Physics Today by Sarmiento & Gruber. The paper was published nearly 20 years ago, so the numbers are a bit out of date, but the underlying messages are still valid. The figure shows that the reservoirs of carbon stored in the land and ocean reservoirs are huge, exceeding that in the atmosphere by factors of 4 and 65 respectively. The carbon that’s recirculated back into the atmosphere isn’t only from land plants and animals. A similar fraction is emitted back from the oceans, which have a longer residence time, and less than 10 percent of that is emitted by mankind’s burning of fossil fuels. The fluxes of carbon dioxide back into the atmosphere from those two dominant sources contain much lower levels of the radiocarbon produced in those nuclear tests, so the atmospheric rate of replenishment for modern carbon isotope is much slower than the rate of replacement for all isotopes. So the fast rate of decay of radiocarbon gives a midsleading picture. Finally, the assumption that “all isotopes behave exactly like normal CO2” is wrong. Plants preferentially recirculate the normal isotope, rather than the heavier isotopes.

To climate scientists, the atmospheric lifetime includes the whole period that the additional CO2 will play a part, and that includes the effects of these short term recyclings with the biosphere. And for CO2 the time scale is indeed hundreds of years.
Incidentally, the main global problem is from CO2, but unlike most developed countries, agricultural producers like New Zealand, which have a high methane (CH4) emissions, have two levers we can pull to solve the problem. We can either decrease our CO2 emissions or (more easily) decrease our CH4 emissions. In a sense we’re lucky, but the agricultural sector – perhaps unfairly - bears the brunt. It also has a much more rapid effect. Its atmospheric lifetime is only a decade. So controlling CH4 buys us time while longer term solutions are found to curb CO2 production, which is the real solution in global terms. On average the GHG emissions of each New Zealand citizen is about three times the global average (partly because of the way CH4 is counted). And global GHG emissions are currently about three times as high as we can afford. So ours are nearly 10 times too high. If any individuals the world need to act, it’s us. Luckily we are, with the very Zero Carbon Bill that the denier was arguing against.
The good news is that the bill has now been passed, and other countries like China, Japan, and USA (when Trump’s failing fiasco falls) are beginning to follow suit.

Hi Richard, I am convinced that our climate has changed and will continue to change but not convinced that greenhouse gas models accurately mimic the cause-effect interactions of all greenhouse gas sources and sinks and the quantities of different contributions, natural and manmade. In particular, arborists and botanists argue that the main contribution to climate change is massive destruction of natural rain forests, wetlands, natural bush and grasslands to make way for commercial farming and grazing. They argue that climate change models published or referenced by IPCC scientists seriously minimize this contribution because research on the part played by photosynthesis and bacteria is lacking and subsequent scientific data for this factor have not been correctly quantified. Can you comment?