Penetrating thoughts
One of my all-time favourite plots. Saved here for safe keeping and easy retrieval ...
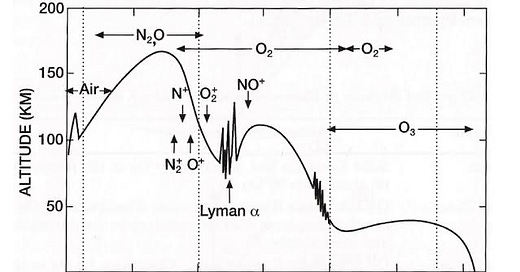
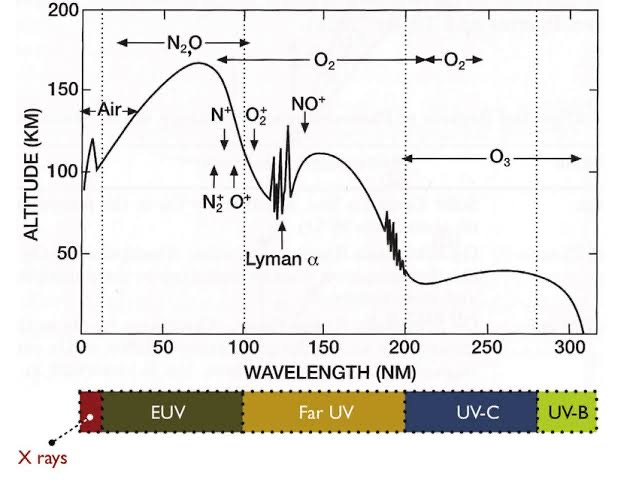
I do like figures, and the one below is one of my favourites. It shows how far UV radiation from the Sun penetrates down into Earth’s atmosphere. The line is the minimum altitude reached at each wavelength out to 320 nm. The radiation that our eyes can see is at longer wavelengths: around 400 to 700 nm. The plot also shows the main absorbers in each wavelength region that cause those reductions. You can find a version of it here (great book, by the way). The original is attributed to L. Herzberg’s, Physics of the Earth's Upper Atmosphere, which was published back in 1965. But I got this version straight from the web.
It’s not that the radiation hits a wall at that line. It’s being attenuated all the time on its path as it penetrates down through the air. The line is just the point when a certain fraction has been lost. The plot shows a short-wavelength limit of radiation reaching the ground at 310 nm, a wavelength about 20 nm longer than the real limit. In fact, the line corresponds to the point when something like 63 percent of the beam has been lost, meaning that about 37 percent (1/e for mathematicians) is still present. That 37 percent is still easily measurable. If you have a good enough instrument - like our UV own spectrometers - you can measure the right down to about 290 nm where irradiances are thousands of times smaller (depending on ozone amount and sun elevation angle).
The plot’s crucial to understanding the causes and effects of ozone depletion because both the atmospheric transmission and the photochemistry that creates and destroys ozone are strong functions of wavelength.
You can see from the plot that UV-C radiation (i.e., radiation in the range from 100 to 280 nm) gets absorbed higher in the atmosphere, within the ozone layer. The most likely UV-C wavelength to make it through would be near 220 nm, but the amount reaching the surface would always be less than those tiny amounts present at 290 nm. So, it clearly demonstrates why you don’t see any UV-C radiation at the surface (despite the ill-informed protestations of some), and why we’re less concerned about the effects at wavelengths longer than 315 nm. I should add here that although ozone does absorb in both the visible and infra-red portions of the spectrum, the absorption strength at those longer wavelengths is much smaller than in the UV region shown above.
Here’s another example of its usefulness. A hot topic of research is the question of whether water vapour from the Tongan eruption earlier this year could have accelerated ozone depletion. It certainly penetrated high enough. Could it thus explain the low ozone amounts we’ve been experiencing in the southern hemisphere ever since those unprecedented amounts of water vapour had been injected high into the stratosphere?
My initial thought was that the molecules of water vapour (H2O) would be split up by UV radiation from the sun (i.e., photolysed) to directly form OH and H. Those reaction products are known to already be major players in ozone depletion, so putting more of them into the stratosphere would certainly decrease ozone. But, after discussions with my colleague, Laura Revell, during a conference a couple of weeks ago in Dunedin, I was reminded that this particular photolysis reaction requires radiation at wavelengths less than 200 nm. Photons from wavelengths longer than that limit don’t have enough energy to break water molecules apart. Looking at the above plot, you can see that the reaction can therefore occur only at altitudes higher than about 40 km, well above the peak of the ozone layer. There is, however, another less direct pathway. The H2O can react directly with atomic oxygen - which is present at lower altitudes within the ozone layer - to form two molecules of OH, which can then go on to catalytically destroy ozone. So the answer is yes: additional ozone depletion is expected following the eruption. And, in the presence of other volcanic aerosol, the ozone depletion would be more rapid because of faster reactions that can occur on the surfaces of those aerosols (i.e., so-called ‘heterogeneous’ reactions). Extended periods of ozone losses seen over large areas after the eruption of Mount Pinatubo in 1991 tells us that these can be important.
And yet another example. The plot also hints at one of the difficulties of satellite-based measurements of the amount of UV reaching Earth’s surface. A common method is for a satellite-based sensor to measure UV radiation originating at the Sun that’s been back-scattered from near Earth’s surface. The amount of ozone is derived from its UV-B absorption due in that return beam. But, as you can in infer from the plot, very little of the incident UV-B radiation penetrates right down to Earth’s surface. Importantly, some of that decrease is due to scattering by molecules of air in the atmosphere (Rayleigh scattering), rather than ozone absorption. At 300 nm, about half the radiation gets scattered long before reaching the surface, even in clean air and for overhead sun. It gets worse when the sun is lower in the sky or under polluted conditions. The mean scattering height gets higher and higher. So to infer the total amount of ozone, you need to make corrections for the portion below that mean scattering height. As it happens, that’s not too difficult because the amount of ozone in those lowest layers is only a small fraction of the total. Most is much higher in the atmosphere, centred at altitudes around 15 to 30 km. But for estimating UV at the surface that lack of knowledge about the lowermost reaches is a big problem. Absorptions of UV radiation in the lowest parts of the atmosphere can be large, especially under polluted conditions. It’s even more problematic when clouds obscure the field of view - as they usually do.
Anyway. It’s good to have the plot tucked away safely here for posterity. .


Richard, what have scientists found concerning Carbon Pentoxide and Carbon Hexoxide in the stratosphere? Like Ozone those rare molecules can be produced from Carbon Dioxide and Oxygen by UV radiation in cryogenic temperatures? I expect increasing levels of atmospheric Carbon Dioxide would cause increasing levels of Carbon Pentoxide and Carbon Hexoxide. Do they absorb UV radiation and offset or aggravate ozone depletion?